What factors contribute to the development of antiparasitic resistance??
Parasite, management, and drug factors all contribute to the development of antiparasitic resistance.
Parasite Factors
Multiple mechanisms may confer resistance to a parasite population including:
- Genetics of the parasite.
- The development of antiparasitic resistance can be due to mutations in a single gene or multiple genes in the parasite’s genome. Resistance development further depends on the genes’ heritability; (ie how much the resistance can be attributed to genetic factors versus environmental factors), and whether the genes are dominant or recessive. Although dominant and recessive genes are passed on at the same rate, resistance due to dominant genes is phenotypically seen sooner in the parasite population than resistance due to recessive genes. This is because the offspring need to inherit only one dominant copy of the gene from one parent to express resistance, rather than two recessive copies (one from each parent).
- Biology of the parasite.
- The parasite’s life cycle, relationship with the host, and number of eggs produced influence how fast resistance develops.
Poor Management Practice
Practices that speed up antiparasitic resistance include:
- Treating too often.
- Each treatment with an anti-parasitic drug eliminates parasites with susceptible genotypes. With increased treatment frequency, there are more opportunities to kill susceptible parasites and leave behind resistant ones. The result is a greater proportion of resistant parasites in the total parasite population.
- Treating the entire herd.
- Treating the entire herd at the same time eliminates the susceptible parasites from all animals at once. This increases the proportion of resistant parasites in the total parasite population.
- Strategic deworming.
- Strategic deworming is treating when most parasites are in the host animal rather than in the environment. Treatment is commonly done at housing, which is beneficial for the animals.
However, after treatment, resistant parasites only are left in the host animal and produce eggs that harbour resistance genes. The host animal then sheds these eggs onto the pasture in the following season. Because the overall numbers of eggs and larvae on the pasture are low at the time of strategic deworming, the proportion of resistant eggs goes up, resulting in an increased frequency of resistance genes in the total parasite population.
- Having inadequate quarantine procedures.
- Newly purchased animals may harbour resistant parasites. If new arrivals are introduced into a flock or herd without following adequate quarantine procedures (such as, prior to introduction, treating new animals with an antiparasitic drug and performing a faecal egg count reduction test to confirm treatment was effective), they may shed eggs from resistant parasites, increasing the frequency of resistance genes on the farm.
- Under-dosing.
- Treating animals with less than the approved dose of an Antiparasitic drug exposes the parasite population to a sub-therapeutic drug level, leading to resistance.
Good Management Practice
Practices that decrease selection pressure for antiparasitic resistance include:
- Dosing with adequate amounts of the antiparasitic drug to avoid underdosing.
- Good practice is to weigh some or all of the group and treat for the heaviest animals in the group. Also ensure dosing gun/syringe is fully calibrated before each treatment group to give accurate dosing volume and further reduce the risk of underdosing
- Choosing the “right” antiparasitic drug.
- This choice is an informed decision, based on diagnostics, such as the faecal egg count reduction test, to determine which drug is most effective against the parasites present on a farm.
By using the most effective drug or drugs, one treatment kills more parasites, leaving behind fewer surviving resistant parasites to pass on resistance genes.
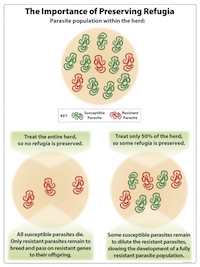
- Preserving refugia.
- See below
- Using antiparasitic drugs along with non-chemical control methods,
- such as good pasture management practices, adequate quarantine procedures, and routine culling of heavy egg shedders.
Drug Factors
The antiparasitic drug’s behaviour both in the animal and in the parasite impacts how quickly resistance develops.
Long acting drugs select for resistance
Long-acting drugs that persist in the animal’s body a long time after initial treatment tend to select for resistance more quickly than short acting drugs.
Long-acting drugs speed up antiparasitic resistance by not only putting selection pressure on the parasites present in the animal at the time of treatment (head selection),but also on parasite larvae ingested after treatment (tail election). Also, as the level of a long-acting drug declines in an animal’s body over time, parasites are exposed to gradually decreasing drug levels, which may accelerate the development of resistance.
Refugia
What is Refugia?
Refugia is the proportion of the total parasite population that isn’t selected for antiparasitic drug treatment—essentially, those parasites that are in “refuge” from the drug. Therefore, there’s no selection pressure on these parasites to develop resistance. Refugia occurs both inside the animal and in the environment and includes:
- Parasites in untreated animals, called host-based refugia;
- Eggs and larvae already on the pasture when the animals are treated, called environmental refugia; and
- Life stages of the parasite that are unaffected by drug treatment, such as some larval stages.
What is the Purpose of Preserving Refugia?
The purpose of preserving refugia is to maintain drug-sensitive (susceptible) parasites. The presence of some drug-sensitive parasites decreases (dilutes) the proportion of resistant parasites within the parasite population on a farm.
What are two strategies to preserve refugia?
Two strategies to preserve refugia are to:
- Leave some animals untreated by limiting treatment to the few animals shedding the most eggs and/or the ones most clinically affected; and
- Deworm when there are more eggs and larvae on the pasture (when environmental refugia is greater). This is the opposite of strategic deworming; where treatment is done when environmental refugia is minimal.
Two ways to leave some animals untreated are to:
- Use targeted selective treatments. Use a highly effective drug and treat only those animals that need treatment, based on the results of faecal egg count reduction tests Consider the 80-20 rule: 80 percent of parasite eggs are shed by 20 percent of the animals in a flock or herd.
- Focus treatment on certain classes of animals. It may be easier to convince producers to not treat certain classes of animals, rather than convince them to not treat a proportion of each class. Certain classes of animals may require treatment with antiparasitic drugs more than others. Allowing some animals, depending on their age and class, to have some parasites isn’t contrary to production goals. Animals don’t have to be completely parasite-free to be healthy and thrive. However, any animal showing clinical signs of parasitism should be treated.
- Low priority classes: Suckling animals, mature horses, and mature lactating dairy cows. These groups of animals can generally be left untreated and still remain healthy due to either (1) their physiologic status for example, suckling animals that aren’t grazing yet, and therefore, are less likely to ingest parasite larvae from the pasture, (although in the tropics suckling lambs and calves must be in high priority class as certain species e.g. some Strongyloides can infect through the skin or orally through milk ) or (2) good pasture management practices.
- Moderate priority classes: Breeding males, first-calf heifers, and mature ewes and does. These groups of animals are maintained in ways that may or may not present risks for parasite transmission. Whether to treat with an antiparasitic drug or not depends more on specific parasite situation on the farm.
- High priority classes: Replacement heifers, feedlot cattle, grazing sheep and goats, and juvenile horses. Because most animals in these groups are young or on pasture, they are at greatest risk for parasitism and likely require treatment with antiparasitic drugs. However, young animals should have sufficient parasite exposure to acquire adequate immunity, requiring a balance between exposing them to parasites and deworming them to prevent clinical parasitism.
Faecal Egg Counts and Faecal Egg Count Reduction Tests
The faecal egg count (FEC) is the traditional method to count parasite eggs in a faecal sample. The faecal egg count reduction test (FECRT) is a mathematical calculation of the reduction in parasite eggs from the pre-treatment FEC to the post-treatment FEC.
Avoid introducing resistant worms – use quarantine treatments
Quarantine treatments are highly necessary to prevent the introduction of AR into a herd or flock The objective of quarantine treatments is to reduce the probability of any AR worms being Introduced onto the farm. (If any resistant worms do survive the quarantine treatment, then their numbers should be so low that the emergence of AR is greatly delayed.) Quarantine should be applied to animals purchased from other farms, and which, which have been grazing on other farms (or common grazing) where the resistance status is unknown or likely to be different from the home farm.
There are three steps in the recommended quarantine protocols:
Step 1 – Treatment
All animals brought onto the farm should be treated with anthelmintics likely to remove all worms – both resistant and susceptible genotypes.
Assume the stock are carrying BZ-resistant, LM-resistant, and possibly ML-resistant parasites, when considering treatments. It is particularly important to try to exclude the rarer genotypes, because it is more likely that these represent a genotype currently absent from the farm. To achieve this, stock should be treated with two anthelmintics
The two treatments should be given sequentially not simultaneously. These products should not be mixed before administration. There is no specific recommendation for a time interval between administration of the two products – it could be as short as a few seconds when two operators with different drench guns/injectors
Step 2 - Holding
Hold animals off pasture for 24-48 hours, until any worm eggs present in the gut have passed out in the faeces.
This time period allows worm eggs produced by worms before treatment to pass out in the faeces. After 24 hours, about 90% of the eggs will have been passed and by 48 hours, 99% will have gone. Animals should have access to feed and water throughout the period that they are held off pasture. Faeces passed in the 24-48 hours post-treatment should not be applied to pastures that will subsequently be grazed by stock. Dispose by incineration, or by application to ground that is not grazed.
Step 3 - Turnout to dirty pasture
Animals should be turned out to pasture contaminated with worm eggs and larvae, to minimise the impact of any worms that survive treatment on the farm’s AR status. This will ensure that any eggs produced by worms before treatment and passed in the faeces, will be diluted by the pre-existent free-living stages on the contaminated pasture. This will have the effect of (a) keeping the introduced resistant genes at a low frequency in the free-living population and (b) encouraging rapid re-infection of introduced stock with home-farm worms, shortening the period when introduced worms are dominant (if a non-persistent drug is used). If contaminated pastures are not available, should remain under restriction (step 2, above) for 72 hours before release onto a small pasture. The efficacy of the quarantine treatment should then be assessed by FEC sampling at least 10 animals, 14 days after treatment. If FECs are more than zero, treatment with a highly efficacious anthelmintic should be repeated until 14-day post-treatment FECs are zero. At that point, the stock can be released onto other farm pastures. The small pasture field contaminated by the eggs of surviving worms should be avoided for grazing until it can be quickly and heavily contaminated by grazing with high FEC sheep from the home flock.
1FDA’s Public Meeting on Antiparasitic Drug Use and Resistance in Ruminants and Equines – An Overview Meeting Dates: March 5 and 6, 2012
2Sustainable Control of Parasites in Sheep Technical manual for Sustainable worm strategies for sheep for vets